Unit Of Measurement Of Heat
Contents
Engineering Units
An overview of the units of measurement used in the Steam and Condensate Loop including temperature, pressure, density, volume, oestrus, piece of work and energy.
Throughout the engineering industries, many different definitions and units have been proposed and used for mechanical and thermal properties.
The problems this caused led to the development of an agreed international system of units (or SI units: Système International d'Unités). In the SI system in that location are seven well-defined base units from which the units of other properties can exist derived, and these will be used throughout this publication.
The SI base units include length (in metres), mass (in kilograms), fourth dimension (in seconds) and temperature (in kelvin). The first three will hopefully need no further caption, while the latter will be discussed in more detail later.
The other SI base of operations units are electric current (in amperes), amount of substance (in moles) and luminous intensity (in candela). These may be familiar to readers with a background in electronics, chemistry and physics respectively, merely take little relevance to steam engineering science nor the contents of The Steam and Condensate Loop.
Table ii.i.ane shows the derived units that are relevant to this bailiwick, all of which should exist familiar to those with any full general engineering groundwork. These quantities have all been assigned special names afterward famous pioneers in the evolution of scientific discipline and engineering.
Table two.1.one Named quantities in derived SI units.
| Quantity | Proper name | Symbol | SI base unit of measurement | Derived unit of measurement |
| Area | foursquare metre | A | m2 | - |
| Volume | cubic metre | V | miii | - |
| Velocity | metre per 2nd | u | grand/s | - |
| Acceleration | metre per second squared | a | m/south2 | - |
| Force | newton | N | kg 1000/sii | J/m |
| Energy | joule | J | kg m2/stwo | N 1000 |
| Pressure or stress | pascal | Pa | kg/m s2 | Northward/m2 |
| Power | watt | W | kg k2/sthree | J/s |
At that place are many other quantities that have been derived from SI base units, which will also be of significance to anyone involved in steam applied science. These are provided in Table two.1.2.
Table two.1.ii Other quantities in derived SI units
| Quantity | SI base of operations unit of measurement | Derived unit |
| Mass density | kg/m3 | kg/m3 |
| Specific volume (5g) | g3/kg | one thousandthree/kg |
| Specific enthalpy (h) | m2/s2 | J/kg |
| Specific heat chapters (cp) | m2/southward2 M | J/kg K |
| Specific entropy | chiliad2/s2 K | J/kg K |
| Heat flowrate | m2 kg/south3 | J/s or W |
| Dynamic viscosity | kg/grand s | N s/m² |
Dot notation
Multiples and submultiples
Table 2.1.3 gives the SI prefixes that are used to form decimal multiples and submultiples of SI units. They allow very large or very small numerical values to be avoided. A prefix attaches directly to the proper name of a unit, and a prefix symbol attaches directly to the symbol for a unit.
In summary: one m metres may be shown as i km, 1 000 yard or 10³ m.
Table ii.one.3 Multiples and submultiples used with SI units
| Multiples | Submultiples | ||||
| Factor | Prefix | Symbol | Factor | Prefix | Symbol |
| 1012 | tera | T | 10-3 | milli | chiliad |
| 109 | giga | Grand | 10-6 | micro | μ |
| xhalf-dozen | mega | 1000 | 10-9 | nano | n |
| x3 | kilo | k | x-12 | pico | P |
Special abbreviations used in steam flowmetering applications
For historical reasons, International Standard ISO 5167 (supersedes BS 1042) which refers to flowmetering, apply the following abbreviations in Table ii.1.4.
Table 2.ane.iv Symbols used in flowmetering applications
| Symbol | Definition | Unit |
| qM | Mass flowrate | kg/s or kg/h |
| qV | Book flowrate | m3/s |
| QI | Liquid flowrate | I/min |
| QS | Gas flowrate at STP | I/min |
| QF | Gas flowrate actual | I/min |
| QE | Equivalent water flowrate | I/min |
| DSouthward | Density of gas at STP | kg/one thousand3 |
| DF | Density of gas actual | kg/m3 |
| PSouthward | Standard pressure level (1.013 bar a) | bar a |
| PF | Actual flow pressure | bar a |
| TS | Standard temperature | °C |
| TF | Bodily flow temperature | °C |
STP - Standard temperature and pressure level
These are the standard atmospheric condition for measurement of the properties of affair. The standard temperature is the freezing point of pure water, 0 °C or 273.xvi °Grand. The standard pressure is the pressure level exerted past a column of mercury (symbol Hg) 760 mm loftier, often designated 760 mm Hg. This pressure is also called one atmosphere and is equal to 1.01325 x 106 dynes per foursquare centimetre, or approximately 14.7 lb per square inch. The density (mass per book) of a gas is usually reported as its value at STP. Properties that cannot be measured at STP are measured under other weather condition; normally the values obtained are then mathematically extrapolated to their values at STP
Symbols
Table two.ane.5 shows the symbols and typical units used in The Steam and Condensate Loop.
Table 2.1.5 Symbols and units of measure used in The Steam and Condensate Loop
| Symbol | Definition | Unit |
| A | Cross sectional surface area of a conduit,for the operating status | thousand² or mm² |
| cP | Specific heat capacity at abiding pressure level | kJ/kg °C or kJ/kg K |
| c5 | Specific heat capacity at constant book | kJ/thou³ °C or kJ/g³ 1000 |
| D | Diameter of the circular cross section of a conduit | m or mm |
| d | Orifice diameter | m or mm |
| g | Dispatch due to gravity | ix.81 grand/s² |
| Hz | Hertz, the unit of measurement of frequency (number of cycles per second) | Hz or kHz |
| J | Joule, the unit of energy | J or kJ |
| Fifty | Length | chiliad |
| 1000 | Molar mass of a fluid | kg/mol |
| N | Newton, the unit of force | North or kN |
| Pa | Pascal, the unit of pressure | Pa or kPa |
| p | Static pressure of a fluid | bar or kPa |
| ∆p | Differential pressure | bar or kPa |
| m | Fundamental unit of length (metre) | one thousand |
| one thousand | Mass | kg |
| ṁ | Mass flowrate | kg/s or kg/h |
| ṁS | Steam mass flowrate | kg/s or kg/h |
| Q | Quantity of rut | kJ |
| Q̇ | Heat transfer rate | kJ/south (kW) |
| R | Radius | 1000 or mm |
| ReD | Reynolds number referred to diameter D | Dimensionless |
| s | Fundamental unit of measurement of time (second) | south |
| Sr | Strouhal number | Dimensionless |
| σ | Stress | Due north/thousand² |
| TSouthward | Steam temperature | K or °C |
| TFifty | Liquid (or production) temperature | G or °C |
| ∆T | Temperature difference or change | K or °C |
| t | Time | s or h |
| u | Velocity of a fluid | m/s |
| μ | Dynamic viscosity of a fluid | Pa southward or cP |
| ν | Kinematic viscosity | cSt |
| ρ | Density of a fluid | kg/m³ |
| V̇ | Book flowrate | 1000³/s or k³/h |
| W | Unit of measurement of energy menstruum (Watt) | W (J/south) |
| V (vg) | Volume (Specific volume) | m³ (m³/kg) |
| H (hg) | Enthalpy (Specific enthalpy) | kJ (kJ/kg) |
| S (sg) | Entropy (Specific entropy) | kJ/K (kJ/kg K) |
| U (ug) | Internal energy (specific internal energy) | kJ (kJ/kg) |
Subscripts used with properties
When using enthalpy, entropy and internal energy, subscripts as shown beneath are used to place the phase, for example:
- Subscript f = Fluid or liquid state, for example hf: liquid enthalpy
- Subscript fg = Modify of state liquid to gas, for case hfg: enthalpy of evaporation
- Subscript g = Total, for example hg: total enthalpy
Note that, by convention, the total heat in superheated steam is signified by h.
It is as well usual, by convention, to signify sample quantities in capital messages, whilst unit of measurement quantities are signified in lower example letters.
For example:
Total enthalpy in a sample of superheated steam H kJ
Specific enthalpy of superheated steam h kJ/kg
Atmosphereature
The temperature scale is used as an indicator of thermal equilibrium, in the sense that any two systems in contact with each other with the same value are in thermal equilibrium.
The Celsius (°C) scale
This is the scale near usually used by the engineer, as it has a convenient (but arbitrary) zero temperature, respective to the temperature at which water will freeze.
The accented or Yard (kelvin) scale
This scale has the same increments every bit the Celsius scale, but has a zero corresponding to the minimum possible temperature when all molecular and atomic motion has ceased. This temperature is often referred to as absolute zilch (0 Yard) and is equivalent to -273.16 °C.
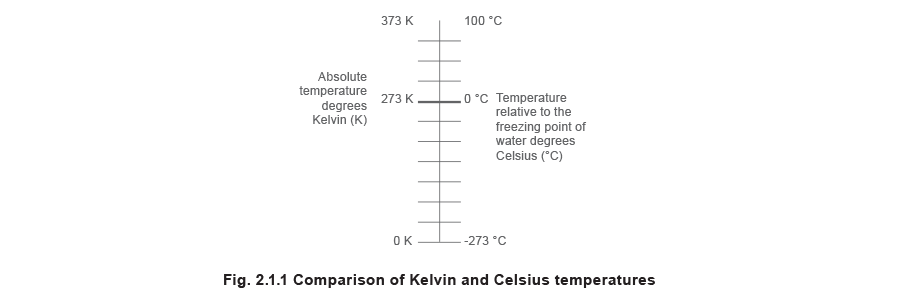

The ii scales of temperature are interchangeable, every bit shown in Figure ii.i.1 and expressed in Equation ii.1.i.
The SI unit of temperature is the kelvin, which is defined as 1 ÷ 273.16 of the thermodynamic temperature of pure water at its triple point (0.01 °C). An explanation of triple indicate is given in Module ii.2.
Almost thermodynamic equations crave the temperature to be expressed in kelvin. Notwithstanding, temperature difference, as used in many heat transfer calculations, may exist expressed in either °C or K. Since both scales have the same increments, a temperature difference of 1 °C has the aforementioned value as a temperature departure of one Chiliad.
Pressure
The SI unit of force per unit area is the pascal (Pa), divers as 1 newton of force per foursquare metre (1 N/m²).
As Pa is such a small unit the kPa (1 kilonewton/m²) or MPa (1 Meganewton/m²) tend to be more advisable to steam engineering.
Nevertheless, probably the most commonly used metric unit for pressure level measurement in steam technology is the bar. This is equal to 105 North/m², and approximates to 1 atmosphere. This unit is used throughout this publication.
Other units often used include lb/in² (psi), kg/cm², atm, in Water and mm Hg. Conversion factors are readily available from many sources.
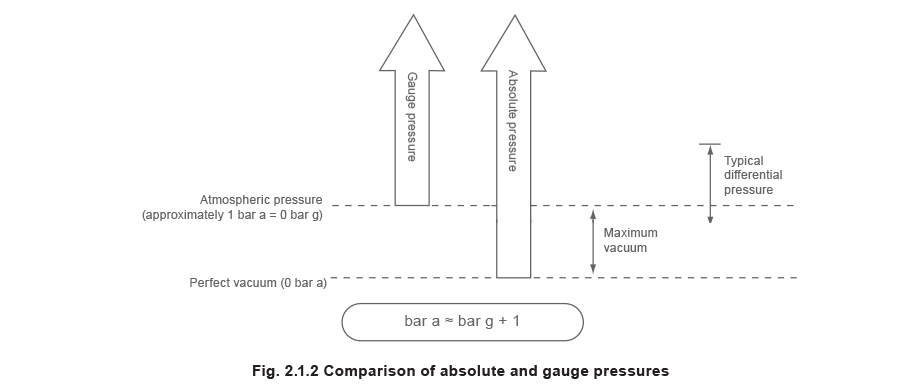
Accented pressure (bar a)
This is the force per unit area measured from the datum of a perfect vacuum i.east. a perfect vacuum has a pressure of 0 bar a.
Judge pressure (bar grand)
This is the pressure measured from the datum of the atmospheric force per unit area. Although in reality the atmospheric force per unit area will depend upon the climate and the height above sea level, a mostly accepted value of 1.013 25 bar a (1 atm) is frequently used. This is the average pressure level exerted by the air of the earth's atmosphere at ocean level.
Gauge pressure = Accented force per unit area - Atmospheric pressure level
Pressures above atmospheric volition e'er yield a positive gauge pressure. Conversely a vacuum or negative force per unit area is the pressure below that of the atmosphere. A pressure of -1 bar m corresponds closely to a perfect vacuum.
Differential pressure level
This is only the difference between two pressures. When specifying a differential pressure, it is not necessary to use the suffixes 'thousand' or 'a' to denote either gauge pressure or absolute pressure level respectively, as the pressure datum signal becomes irrelevant.
Therefore, the departure between two pressures will accept the same value whether these pressures are measured in guess pressure or accented pressure, every bit long as the two pressures are measured from the same datum.
Density and specific volume
The density (ρ) of a substance can exist defined as its mass (m) per unit volume (V). The specific volume (vg) is the volume per unit mass and is therefore the inverse of density. In fact, the term 'specific' is generally used to denote a property of a unit mass of a substance (see Equation two.ane.2).
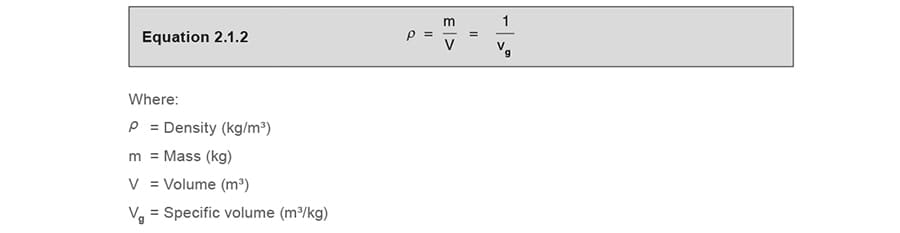
The SI units of density (ρ ) are kg/m³, conversely, the units of specific volume (vg) are m³/kg.
Another term used equally a mensurate of density is specific gravity. It is a ratio of the density of a substance (ρs) and the density of pure water (ρw) at standard temperature and pressure (STP).
This reference condition is commonly defined as being at atmospheric pressure and 0°C. Sometimes it is said to be at 20°C or 25°C and is referred to as normal temperature and pressure (NTP).

The density of water at these conditions is approximately 1 000 kg/yard³. Therefore substances with a density greater than this value will have a specific gravity greater than 1, whereas substances with a density less than this will have a specific gravity of less than one.
Since specific gravity is a ratio of two densities, it is a dimensionless variable and has no units. Therefore in this case the term specific does not betoken information technology is a property of a unit mass of a substance. Specific gravity is also sometimes known every bit the relative density of a substance.
Estrus, piece of work and energy
Free energy is sometimes described as the ability to exercise work. The transfer of free energy by means of mechanical motion is called work. The SI unit of measurement for work and energy is the joule, defined equally 1 North m.
The amount of mechanical work carried out can be determined by an equation derived from Newtonian mechanics:
Piece of work = Force x Displacement
It can also exist described as the product of the practical pressure and the displaced volume:
Piece of work = Applied pressure x Displaced book
Example 2.one.i
An applied pressure of 1 Pa (or one Due north/m²) displaces a book of ane k³. How much work has been done?
Work washed = 1 N/g² ten one 1000³ = ane N m (or 1 J)
The benefits of using SI units, as in the above instance, is that the units in the equation actually cancel out to requite the units of the product.
The experimental observations of J. P. Joule established that there is an equivalence betwixt mechanical energy (or work) and estrus. He institute that the aforementioned amount of free energy was required to produce the same temperature rise in a specific mass of water, regardless of whether the free energy was supplied as heat or work.
The total energy of a organization is composed of the internal, potential and kinetic energy. The temperature of a substance is directly related to its internal energy (ug). The internal energy is associated with the movement, interaction and bonding of the molecules within a substance. The external energy of a substance is associated with its velocity and location, and is the sum of its potential and kinetic free energy.
The transfer of energy as a result of the deviation in temperature lonely is referred to as estrus flow. The watt, which is the SI unit of measurement of ability, can be defined as 1 J/south of oestrus flow.
Other units used to quantify heat energy are the British Thermal Unit (Btu: the corporeality of rut to heighten one lb of water by 1 °F) and the kilocalorie (the amount of rut to raise 1 kg of water by 1 °C).
Conversion factors are readily bachelor from numerous sources.
Specific enthalpy
This is the term given to the total energy, due to both pressure and temperature, of a fluid (such equally water or steam) at any given fourth dimension and status. More than specifically it is the sum of the internal energy and the piece of work done by an applied pressure (equally in Instance 2.1.1).
The basic unit is the joule (J). Since one joule represents a very pocket-sized amount of energy, it is usual to utilize kilojoules (kJ = 1 000 joules).
The specific enthalpy is a measure out of the total free energy of a unit mass, and its units are ordinarily kJ/kg.
Specific estrus capacity
The enthalpy of a fluid is a function of its temperature and force per unit area. The temperature dependence of the enthalpy tin can be institute by measuring the ascent in temperature caused by the flow of heat at constant pressure. The constant-pressure heat capacity cP, is a measure of the change in enthalpy at a particular temperature.
Similarly, the internal free energy is a function of temperature and specific volume. The constant volume heat capacity cv, is a measure of the change in internal energy at a particular temperature and abiding book.
Because the specific volumes of solids and liquids are mostly smaller, then unless the force per unit area is extremely loftier, the work washed by an applied pressure tin be neglected. Therefore, if the enthalpy tin can exist represented past the internal energy component alone, the constant-volume and constant-pressure heat capacities can be said to be equal.
Therefore, for solids and liquids: cP ≈ cv
Another simplification for solids and liquids assumes that they are incompressible, so that their volume is simply a function of temperature. This implies that for incompressible fluids the enthalpy and the heat capacity are also merely functions of temperature.
The specific heat capacity represents the corporeality of energy required to enhance one kg past 1 °C, and tin can be thought of equally the ability of a substance to absorb heat. Therefore the SI units of specific heat chapters are kJ/kg Thou (kJ/kg °C). Water has a big specific heat capacity (4.19 kJ/kg °C) compared with many fluids, which is why both water and steam are considered to exist good carriers of rut.
The amount of heat energy required to enhance the temperature of a substance tin exist adamant from Equation 2.1.iv.
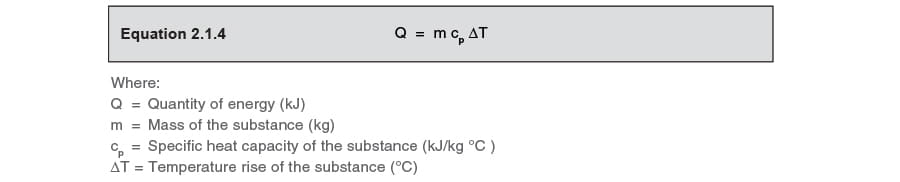
This equation shows that for a given mass of substance, the temperature ascent is linearly related to the corporeality of heat provided, assuming that the
specific heat capacity is constant over that temperature range.
Example 2.1.2
Consider a quantity of water with a volume of 2 litres, raised from a temperature of 20 °C to 70 °C.
At atmospheric pressure, the density of water is approximately 1 000 kg/m³. As in that location are 1 000 litres in i thousand³, so the density tin can be expressed equally 1 kg per litre (1 kg/l). Therefore the mass of the water is 2 kg.
The specific heat capacity for water tin can exist taken every bit 4.nineteen kJ/kg °C over low ranges of temperature.
Therefore: Q =two kg x 4.nineteen kJ/kg °C x (70 - 20) °C = 419 kJ
If the h2o was then cooled to its original temperature of 20 °C, it would as well release this amount of free energy in the cooling application.
Entropy (S)
Entropy is a measure of the caste of disorder within a system. The greater the degree of disorder, the higher the entropy. The SI units of entropy are kJ/kg K (kJ/kg °C).
In a solid, the molecules of a substance adapt themselves in an orderly structure. Every bit the substance changes from a solid to a liquid, or from a liquid to a gas, the arrangement of the molecules becomes more disordered as they begin to movement more than freely. For any given substance the entropy in the gas stage is greater than that of the liquid phase, and the entropy in the liquid phase is more than in the solid stage.
One characteristic of all natural or spontaneous processes is that they proceed towards a country of equilibrium. This can be seen in the 2nd law of thermodynamics, which states that rut cannot pass from a colder to a warmer body.
A alter in the entropy of a system is acquired past a change in its heat content, where the change of entropy is equal to the heat change divided by the boilerplate accented temperature, Equation 2.1.5.
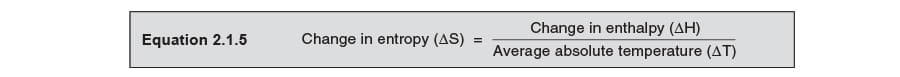

To look at this in further item, consider the following examples:
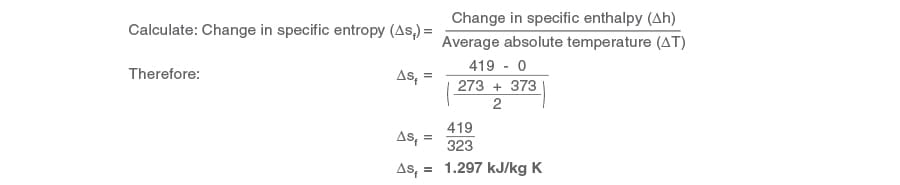
Example 2.1.3
A process raises one kg of water from 0 to 100°C (273 to 373 Yard) under atmospheric conditions.
Specific enthalpy at 0°C (hf) = 0 kJ/kg (from steam tables)
Specific enthalpy of h2o at 100°C (hf) = 419 kJ/kg (from steam tables)
Calculate the change in specific entropy
Since this is a modify in specific entropy of water, the symbol 's' in Equation two.ane.6 takes the suffix 'f' to become sf.
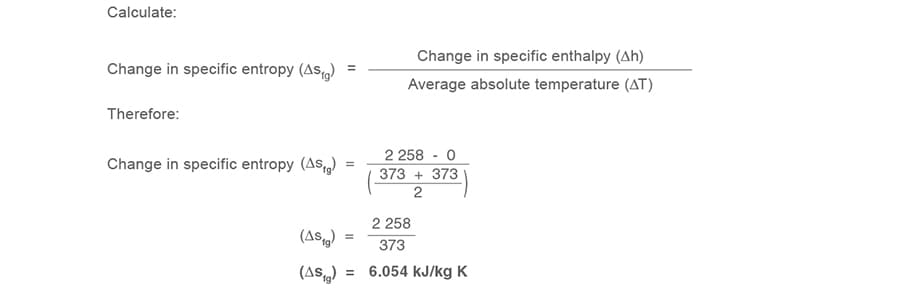
Case 2.1.4
A process changes 1 kg of water at 100°C (373 K) to saturated steam at 100°C (373 K) nether atmospheric conditions.
Calculate the change in specific entropy of evaporation
Since this is the entropy involved in the change of state, the symbol 's' in Equation 2.1.6 takes the suffix 'fg' to become sfg.
Specific enthalpy of evaporation
of steam at 100°C (373 K) (hfg) = 2 258 kJ/kg (from steam tables)
Specific enthalpy of evaporation
of water at 100°C (373 K) (hfg) = 0 kJ/kg (from steam tables)
The total change in specific entropy from water at 0 °C to saturated steam at 100 °C is the sum of the modify in specific entropy for the water, plus the change of specific entropy for the steam, and takes the suffix 'g' to become the total change in specific entropy sg.
Therefore

Example 2.1.five
A process superheats ane kg of saturated steam at atmospheric force per unit area to 150°C (423 K). Make up one's mind the modify in entropy.
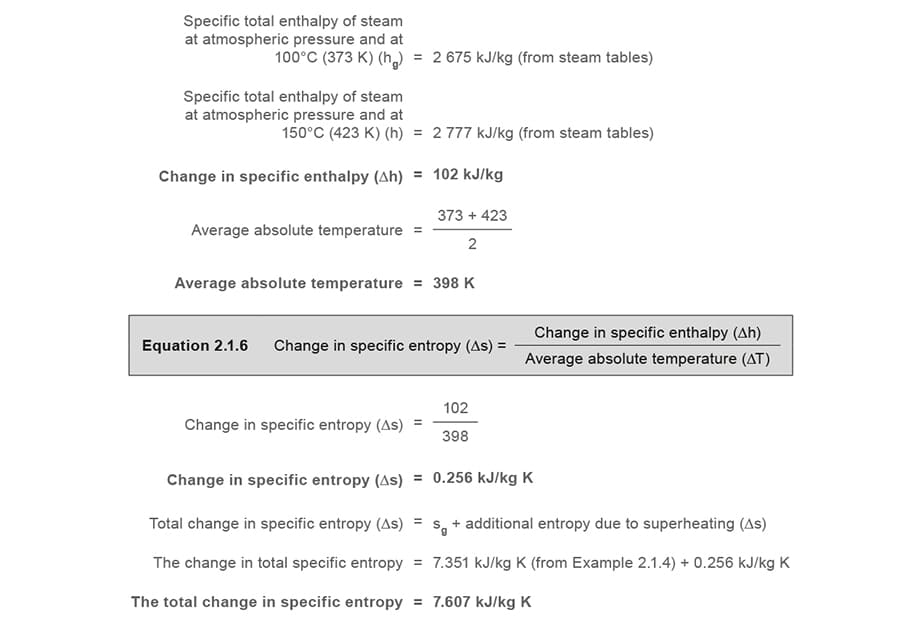
As the entropy of saturated water is measured from a datum of 0.01 °C, the entropy of water at 0 °C can, for practical purposes, be taken equally zero. The total change in specific entropy in this example is based on an initial water temperature of 0 °C, and therefore the final result happens to be very much the same as the specific entropy of steam that would be observed in steam tables at the final status of steam at atmospheric pressure and 150 °C.
Entropy is discussed in greater particular in Module 2.15, Entropy - A Basic Understanding, and in Module 2.xvi, Entropy - Its Practical Employ.
Unit Of Measurement Of Heat,
Source: https://www.spiraxsarco.com/learn-about-steam/steam-engineering-principles-and-heat-transfer/engineering-units
Posted by: stantonsittoss.blogspot.com


0 Response to "Unit Of Measurement Of Heat"
Post a Comment